Introduction
Epigenetics is a branch of biology that delves into the study of heritable changes in gene expression or cellular phenotype that do not involve alterations to the underlying DNA sequence. While traditional genetics focuses on the sequence of nucleotides in the DNA, epigenetics examines how environmental factors, lifestyle choices, and even behaviors can influence gene activity without changing the genetic code itself. This fascinating field has transformed our understanding of biology, revealing that genetic expression is not solely governed by the DNA sequence but is also shaped by a variety of complex, reversible modifications.
Epigenetic changes can be stable across cell generations, and sometimes, they can even be passed down to offspring, affecting health and development across generations. With implications for cancer, neurological disorders, mental health, aging, and even evolutionary biology, epigenetics plays a pivotal role in understanding biological processes and their connection to the environment. In this study material, we will explore the key mechanisms of epigenetic regulation, its role in health and disease, and its potential applications in medicine and beyond.
1. What is Epigenetics?
Epigenetics is derived from the Greek word “epi,” meaning “above” or “over,” and genetics, which refers to the study of genes. Thus, epigenetics refers to processes that regulate gene activity and expression “above” or beyond the DNA sequence. These regulatory mechanisms do not alter the DNA itself but instead influence how genes are turned on or off, how they are expressed, and how they interact with one another within the genome.
Unlike genetic changes, which are fixed and passed down from one generation to the next through mutations in the DNA sequence, epigenetic modifications are reversible and can be influenced by external factors such as diet, stress, toxins, and environmental exposures. They are an essential part of how organisms adapt to their environment and can have lasting effects on health and disease.
2. Key Mechanisms of Epigenetic Regulation
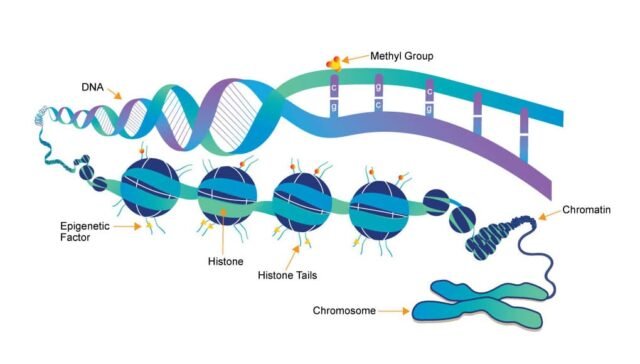
Epigenetic regulation is primarily controlled through three main mechanisms: DNA methylation, histone modification, and non-coding RNA regulation. These processes work in concert to influence gene expression and maintain cellular identity.
2.1 DNA Methylation
DNA methylation is one of the most studied and well-understood epigenetic modifications. It involves the addition of a methyl group (–CH₃) to the DNA molecule, typically at the carbon 5 position of the cytosine base. This modification usually occurs in regions of the genome known as CpG islands, which are stretches of DNA where cytosine is followed by guanine.
Methylation of CpG sites in the promoter region of a gene typically silences its expression, as the methyl group inhibits the binding of transcription factors and other necessary proteins for gene activation. Conversely, the absence of methylation in these regions allows for gene expression. DNA methylation is crucial for normal development, gene imprinting, and X-chromosome inactivation.
2.2 Histone Modification
Histones are proteins around which DNA is wrapped to form a structure known as chromatin. These histones can be chemically modified in several ways, including acetylation, methylation, phosphorylation, and ubiquitination. These modifications can alter the structure of chromatin, making it more or less accessible for transcription.
- Histone Acetylation: This modification typically leads to gene activation. Acetyl groups are added to the lysine residues on histones, causing the chromatin to loosen and allowing for easier access to DNA for transcription.
- Histone Methylation: This can either activate or repress gene expression, depending on the specific context and location of the modification. Methylation of histones at certain sites can lead to a more compact chromatin structure, resulting in gene silencing, while methylation at other sites can activate gene expression.
- Histone Phosphorylation: Often associated with processes such as DNA repair and cell division, histone phosphorylation can also influence gene expression by altering chromatin structure.
2.3 Non-Coding RNAs
Non-coding RNAs (ncRNAs) are RNA molecules that do not encode proteins but play a crucial role in regulating gene expression at the transcriptional and post-transcriptional levels. The most well-known types of ncRNAs involved in epigenetic regulation are microRNAs (miRNAs) and long non-coding RNAs (lncRNAs).
- miRNAs: These small RNA molecules can bind to messenger RNAs (mRNAs) and prevent their translation into proteins, thereby silencing gene expression. miRNAs are involved in many biological processes, including development, differentiation, and disease progression.
- lncRNAs: These longer RNA molecules regulate gene expression by interacting with chromatin, modifying histones, or even guiding enzymes to specific genomic regions. LncRNAs can have both activating and repressive effects on gene expression, playing a significant role in cellular identity and the response to external signals.
3. Epigenetics in Development
One of the most remarkable aspects of epigenetics is its role in development. From the fertilized egg to a fully developed organism, epigenetic changes guide the process of differentiation, where unspecialized cells become specialized in structure and function. Epigenetic mechanisms control which genes are turned on and off in specific cells, ensuring that each cell in the body carries out its specific function.
For example, during early development, stem cells are pluripotent, meaning they have the potential to differentiate into any cell type. Epigenetic changes, such as DNA methylation and histone modifications, help to “lock in” these differentiated states, ensuring that once a cell becomes a muscle cell or a nerve cell, it remains that type of cell throughout its life.
Epigenetic regulation also plays a key role in processes such as:
- X-Chromosome Inactivation: In female mammals, one of the two X chromosomes in each cell is randomly inactivated early in development, a process regulated by epigenetic mechanisms. This ensures that females, like males, have one functional X chromosome in each cell.
- Imprinting: Some genes are expressed in a parent-of-origin-dependent manner, meaning they are only expressed if inherited from one parent and not the other. This is regulated by epigenetic modifications, such as DNA methylation, at the imprinted loci.
4. Epigenetics and Disease
Epigenetic changes have profound implications for health and disease. Unlike genetic mutations, which involve changes in the DNA sequence, epigenetic modifications can be reversible and influenced by environmental factors. As such, they provide a potential target for therapeutic interventions.
4.1 Cancer
One of the most well-known examples of the role of epigenetics in disease is cancer. Abnormal epigenetic modifications can lead to the activation of oncogenes or the silencing of tumor suppressor genes. For instance, hypermethylation of the promoter region of a tumor suppressor gene like p16INK4a can silence its expression, promoting uncontrolled cell growth and tumorigenesis.
Epigenetic therapies are being developed to reverse these changes. For example, drugs that inhibit DNA methylation (such as 5-aza-2′-deoxycytidine) or histone deacetylation (such as vorinostat) can reactivate silenced tumor suppressor genes, potentially offering new avenues for cancer treatment.
4.2 Neurological Disorders
Epigenetic modifications are also involved in various neurological disorders, including Alzheimer’s disease, schizophrenia, and autism. Environmental factors such as stress, toxins, and diet can cause changes in DNA methylation and histone modification patterns, affecting the expression of genes involved in brain function, memory, and behavior.
For instance, studies have shown that early-life stress can lead to epigenetic changes in genes related to stress response and mental health. Understanding these changes opens the door to potential epigenetic therapies that could target specific genes and pathways involved in these disorders.
4.3 Cardiovascular Disease and Metabolic Disorders
Epigenetic changes have been implicated in a range of cardiovascular diseases and metabolic disorders, including obesity, diabetes, and hypertension. These conditions are influenced by both genetic factors and environmental exposures, such as diet and physical activity, which can lead to epigenetic modifications that affect the expression of genes involved in metabolism and cardiovascular function.
5. Epigenetic Inheritance and Environmental Influence
One of the most intriguing aspects of epigenetics is the concept of epigenetic inheritance, which suggests that some epigenetic changes can be passed down from one generation to the next. These changes are not encoded in the DNA sequence but rather in the epigenetic marks that regulate gene expression.
For example, studies have shown that environmental exposures, such as diet or toxins, can lead to epigenetic changes in germline cells (sperm or eggs), which can be inherited by offspring. This concept challenges the traditional view of inheritance, suggesting that traits influenced by environmental factors can be passed down across generations through epigenetic mechanisms.
6. The Future of Epigenetics
The study of epigenetics is still in its early stages, and there is much to learn about the complexities of gene regulation and its implications for health and disease. As research progresses, we can expect to see new breakthroughs in personalized medicine, where epigenetic profiles are used to tailor treatments based on an individual’s genetic and epigenetic makeup.
The potential for epigenetic therapies is vast, offering the possibility of reversing or modifying epigenetic changes associated with various diseases. Moreover, the field of epigenetics may also offer insights into aging, evolution, and the development of new treatments for a wide range of conditions.
Conclusion
Epigenetics represents a frontier in biological research, offering new insights into gene regulation, disease, and inheritance. By examining the molecular mechanisms that control gene expression without altering the DNA sequence itself, we gain a deeper understanding of how our environment, lifestyle, and experiences influence our biology. As the field of epigenetics continues to evolve, it holds promise for revolutionizing medicine, offering new therapies for conditions ranging from cancer to neurological disorders, and even providing a deeper understanding of human evolution and development.