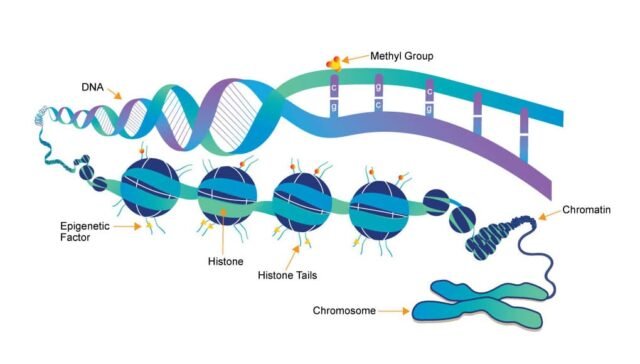
1. What is epigenetics, and how does it differ from genetics?
Answer: Epigenetics refers to the study of changes in gene expression or cellular phenotype that do not involve alterations to the underlying DNA sequence. These changes can be influenced by various factors such as environmental influences, lifestyle, and developmental stages. Unlike genetics, which focuses on mutations or changes in the DNA sequence, epigenetics deals with modifications that regulate gene activity without altering the genetic code itself. These modifications include DNA methylation, histone modification, and non-coding RNA molecules. Epigenetic changes can be reversible and can impact an organism’s development, health, and disease susceptibility.
2. Explain the role of DNA methylation in gene regulation.
Answer: DNA methylation involves the addition of a methyl group (CH3) to the 5′ carbon of a cytosine nucleotide, typically within CpG dinucleotides. This modification generally leads to gene silencing by inhibiting the binding of transcription factors or by recruiting proteins that condense the chromatin, making it less accessible for transcription. DNA methylation is a key epigenetic mechanism for regulating gene expression in normal cellular processes, such as development, differentiation, and X-inactivation. Aberrant DNA methylation can lead to diseases like cancer, where the hypermethylation of tumor suppressor genes results in their silencing.
3. Describe how histone modifications affect gene expression.
Answer: Histones are proteins that package DNA into a compact structure called chromatin. These proteins can undergo various post-translational modifications, such as acetylation, methylation, phosphorylation, and ubiquitination, which play a crucial role in regulating gene expression. Histone acetylation, typically associated with gene activation, neutralizes the positive charge on histones, loosening their interaction with negatively charged DNA and making the chromatin structure more accessible for transcription. In contrast, histone methylation can either activate or repress gene expression depending on the specific site of methylation. For example, methylation on lysine 4 of histone H3 (H3K4me3) is linked to active transcription, whereas methylation on lysine 27 (H3K27me3) is associated with gene repression.
4. What is the significance of non-coding RNAs in epigenetic regulation?
Answer: Non-coding RNAs (ncRNAs) are RNA molecules that do not code for proteins but play essential roles in regulating gene expression. One of the most studied classes of ncRNAs in epigenetics is microRNAs (miRNAs), which can inhibit gene expression by binding to complementary mRNA molecules, preventing their translation. Long non-coding RNAs (lncRNAs) also participate in epigenetic regulation by recruiting chromatin-modifying complexes to specific genomic regions. For example, the lncRNA XIST is involved in X-inactivation, where it coats the X chromosome in females, leading to the transcriptional silencing of one of the X chromosomes. These ncRNAs regulate gene expression at various levels, including transcription, mRNA stability, and translation.
5. Discuss the role of epigenetics in X-inactivation.
Answer: X-inactivation is an epigenetic process that occurs in female mammals to balance the dosage of X-linked genes between males (who have one X chromosome) and females (who have two X chromosomes). One of the X chromosomes in each female cell is randomly inactivated early in development, resulting in a mosaic of cells where some have the paternal X inactivated and others have the maternal X inactivated. The process is mediated by the non-coding RNA XIST, which is transcribed from the X chromosome that will be inactivated. XIST RNA coats the chromosome, recruiting chromatin-modifying proteins that induce DNA methylation and histone modifications, leading to the silencing of the X chromosome.
6. What is the concept of epigenetic inheritance, and how is it different from genetic inheritance?
Answer: Epigenetic inheritance refers to the transmission of epigenetic marks from one generation to the next without changes to the underlying DNA sequence. This inheritance can affect traits and gene expression patterns in offspring, which can be influenced by environmental factors such as diet, stress, and toxins. Unlike genetic inheritance, which involves the transmission of specific DNA sequences (mutations), epigenetic inheritance involves the transmission of epigenetic marks such as DNA methylation or histone modifications that regulate gene activity. Epigenetic inheritance can be temporary or heritable, and in some cases, it may influence an individual’s phenotype without altering the genetic code.
7. Explain how epigenetic modifications contribute to human disease.
Answer: Epigenetic modifications can contribute to various human diseases, including cancer, neurological disorders, and cardiovascular diseases, by disrupting normal gene expression patterns. In cancer, for example, the hypermethylation of tumor suppressor genes can lead to their silencing, promoting uncontrolled cell division. On the other hand, the hypomethylation of oncogenes may result in their activation. In neurological disorders like Rett syndrome, mutations in genes that regulate epigenetic processes (such as the MeCP2 gene) can lead to cognitive impairments. Epigenetic changes can also affect the regulation of genes involved in inflammation, apoptosis, and immune responses, contributing to diseases like autoimmune disorders and cardiovascular diseases.
8. What is genomic imprinting, and how does it work?
Answer: Genomic imprinting is an epigenetic phenomenon where the expression of certain genes depends on the parent of origin. In imprinted genes, only one allele (either the maternal or paternal copy) is expressed, while the other allele is silenced. This silencing is typically achieved through DNA methylation or histone modifications during gamete formation. Imprinting disorders, such as Prader-Willi syndrome and Angelman syndrome, occur when the imprinted gene(s) are inherited from the wrong parent or when epigenetic marks are erased or mutated. Genomic imprinting plays a crucial role in development, especially in regulating the growth of the embryo and the placenta.
9. Discuss the concept of “epigenetic reprogramming” and its importance in stem cell biology.
Answer: Epigenetic reprogramming refers to the resetting of the epigenetic landscape of a cell, allowing it to acquire a new cellular identity. In stem cell biology, this concept is important because it enables somatic cells to be reprogrammed into induced pluripotent stem cells (iPSCs) by altering their epigenetic marks. Reprogramming involves the addition of specific transcription factors that induce changes in DNA methylation and histone modifications, reverting the cells to a pluripotent state similar to embryonic stem cells. This reprogramming has significant implications for regenerative medicine, as it provides a potential source of patient-specific cells for therapeutic purposes.
10. How do environmental factors influence epigenetic modifications?
Answer: Environmental factors such as diet, stress, toxins, and exposure to chemicals can influence epigenetic modifications, which can, in turn, affect gene expression and contribute to disease. For example, a high-fat diet can lead to changes in DNA methylation and histone modifications that affect the expression of genes involved in metabolism, potentially contributing to obesity and diabetes. Similarly, prenatal exposure to environmental toxins, such as bisphenol A (BPA), has been shown to alter DNA methylation patterns in offspring, potentially increasing their risk for diseases like cancer. These epigenetic changes can be passed on to future generations, highlighting the long-term impact of environmental exposures.
11. Explain the role of epigenetic changes in aging.
Answer: Epigenetic changes play a significant role in the aging process by regulating the expression of genes involved in cell proliferation, apoptosis, DNA repair, and stress responses. As individuals age, there is a gradual accumulation of epigenetic changes such as DNA methylation and histone modifications that can lead to the silencing of genes that promote cell survival and repair. Additionally, these changes may affect the immune system, reducing its ability to respond to infections and contributing to age-related diseases like Alzheimer’s and cardiovascular disease. The concept of “epigenetic clocks,” which measure the accumulation of epigenetic changes over time, is being explored as a potential tool for assessing biological age.
12. What is the significance of histone variants in gene regulation?
Answer: Histone variants are alternative forms of the core histone proteins (H2A, H2B, H3, and H4) that can replace the standard histones in chromatin. These variants often have specialized roles in regulating gene expression and chromatin structure. For example, the histone variant H2A.Z is associated with regions of active gene transcription and is thought to play a role in gene activation and chromatin remodeling. Variants such as H3.3 are incorporated into chromatin during transcription and replication, maintaining chromatin accessibility and promoting gene expression. The presence of specific histone variants can influence the transcriptional activity of genes by altering the structure and dynamics of chromatin.
13. What is the role of RNA methylation in epigenetics?
Answer: RNA methylation involves the addition of methyl groups to RNA molecules, particularly mRNA, and plays an important role in regulating gene expression and RNA processing. The most common type of RNA modification is the methylation of adenosine residues, forming N6-methyladenosine (m6A). m6A modification affects RNA splicing, stability, and translation, and is crucial for regulating cellular processes such as differentiation, stress response, and circadian rhythms. Dysregulation of RNA methylation has been linked to various diseases, including cancer and neurological disorders. The enzymes that add and remove these methyl groups serve as potential targets for therapeutic interventions.
14. Explain how epigenetics contributes to the concept of “nature versus nurture.”
Answer: The concept of “nature versus nurture” refers to the debate over the relative contributions of genetic inheritance (“nature”) and environmental factors (“nurture”) in shaping an individual’s phenotype. Epigenetics bridges this gap by providing a mechanism through which environmental factors can influence gene expression and contribute to an individual’s traits and health outcomes without altering the DNA sequence. Epigenetic modifications, such as DNA methylation and histone modifications, can be influenced by factors like diet, stress, and toxins, and these changes can, in some cases, be passed on to future generations. This highlights how nurture (environmental influences) can interact with nature (genetic factors) to shape an individual’s phenotype.
15. What are induced pluripotent stem cells (iPSCs), and how are they related to epigenetics?
Answer: Induced pluripotent stem cells (iPSCs) are somatic cells that have been reprogrammed back to a pluripotent state by the introduction of specific transcription factors, such as Oct4, Sox2, Klf4, and c-Myc. This reprogramming involves significant changes in the cell’s epigenetic landscape, including alterations in DNA methylation patterns and histone modifications, which reset the cell to a state where it can differentiate into any type of cell in the body. iPSCs are an important tool in regenerative medicine, as they can be derived from a patient’s own cells and used for personalized therapies. The ability to reprogram somatic cells into iPSCs demonstrates the reversible nature of epigenetic changes.
16. How does epigenetic reprogramming occur during fertilization and early development?
Answer: Epigenetic reprogramming during fertilization and early development involves the resetting of epigenetic marks in the sperm and egg cells to enable the development of a new organism. After fertilization, the DNA of both the sperm and egg undergoes demethylation, a process that erases many of the epigenetic modifications that had been established in the gametes. This reset ensures that the resulting zygote can develop into a pluripotent cell capable of giving rise to all cell types. During early development, the zygote’s epigenetic landscape is further reprogrammed through DNA methylation, histone modifications, and non-coding RNA activity to establish the patterns of gene expression necessary for cell differentiation and tissue formation.
17. What is the role of epigenetics in cancer therapy?
Answer: Epigenetics plays a significant role in cancer because aberrant epigenetic modifications, such as DNA hypermethylation of tumor suppressor genes and hypomethylation of oncogenes, can drive cancer progression. Understanding these changes opens up new avenues for cancer therapy. Epigenetic therapies aim to reverse abnormal DNA methylation and histone modifications to restore normal gene expression. For example, drugs that inhibit DNA methyltransferases (DNMTs) or histone deacetylases (HDACs) are being investigated for their potential to reactivate silenced tumor suppressor genes or enhance the effectiveness of traditional chemotherapies. Additionally, epigenetic biomarkers are being developed for cancer diagnosis, prognosis, and treatment monitoring.
18. Explain the concept of “epigenetic memory” and its implications for development.
Answer: Epigenetic memory refers to the ability of cells to “remember” specific patterns of gene expression and maintain these patterns across cell divisions, even in the absence of the original stimulus. This memory is primarily mediated through stable epigenetic modifications, such as DNA methylation and histone modifications, which are inherited during cell division. Epigenetic memory is important during development, as it allows cells to retain their differentiated state after cell division. For example, once a stem cell differentiates into a muscle cell, it “remembers” its identity through epigenetic marks, ensuring that it continues to express muscle-specific genes. Epigenetic memory also plays a role in long-term cellular responses to environmental cues.
19. How does the field of epigenetics influence the understanding of mental health disorders?
Answer: Epigenetics has had a profound impact on the understanding of mental health disorders by highlighting the role of gene-environment interactions in the development of conditions like depression, schizophrenia, and bipolar disorder. Environmental factors such as early childhood trauma, stress, or substance abuse can lead to epigenetic changes, like altered DNA methylation or histone modification patterns, which affect the expression of genes involved in brain function and emotional regulation. These epigenetic modifications can persist long after the environmental stressor has passed, contributing to the development and persistence of mental health disorders. Understanding the epigenetic mechanisms underlying mental health disorders has led to the development of potential therapeutic strategies, such as the use of epigenetic drugs to modify gene expression.
20. What is the role of epigenetics in aging and age-related diseases?
Answer: Epigenetic changes accumulate over time and play a crucial role in aging and age-related diseases. As individuals age, alterations in DNA methylation, histone modifications, and non-coding RNA expression can lead to changes in gene expression that contribute to the aging process. These epigenetic changes can affect genes involved in cell cycle regulation, DNA repair, and inflammation, leading to cellular senescence, impaired tissue regeneration, and an increased risk of age-related diseases such as Alzheimer’s disease, cardiovascular disease, and cancer. Epigenetic clocks, which measure the accumulation of epigenetic marks over time, are being used to assess biological age and predict the risk of age-related diseases.
21. What is the relationship between nutrition and epigenetics?
Answer: Nutrition plays a critical role in shaping the epigenome, influencing gene expression through various mechanisms such as DNA methylation, histone modification, and non-coding RNA activity. Certain nutrients, such as folate, B vitamins, and polyphenols, can serve as donors of methyl groups for DNA methylation, affecting gene expression. For example, a diet rich in folate can influence the methylation status of genes involved in cell growth and development. Conversely, nutrient deficiencies or imbalances, such as a lack of methyl donors, can lead to abnormal epigenetic modifications and contribute to diseases like cancer, metabolic disorders, and cardiovascular disease. Epigenetic modifications induced by diet can also be passed on to future generations, highlighting the importance of proper nutrition in maintaining health.
22. What are epigenetic drugs, and how do they work?
Answer: Epigenetic drugs are therapeutic agents designed to modify epigenetic marks, such as DNA methylation and histone modifications, to restore normal gene expression patterns. These drugs aim to reverse abnormal epigenetic changes associated with diseases like cancer, neurological disorders, and cardiovascular disease. For example, DNA methyltransferase inhibitors (e.g., azacitidine) can reactivate silenced tumor suppressor genes by blocking the addition of methyl groups to DNA. Histone deacetylase inhibitors (e.g., vorinostat) can restore acetylation marks on histones, enhancing gene expression. Epigenetic drugs offer a novel approach to treating diseases by targeting the epigenome, rather than the genetic code itself.
23. What is the impact of smoking on epigenetics?
Answer: Smoking has a significant impact on the epigenome, influencing DNA methylation and histone modifications in ways that can promote disease. Cigarette smoke contains numerous toxic chemicals that can alter the epigenetic regulation of genes involved in inflammation, DNA repair, and cell cycle control. For example, smoking has been shown to cause the hypermethylation of tumor suppressor genes, silencing their expression and increasing the risk of cancer. Additionally, smoking-induced epigenetic changes can be passed on to offspring, potentially contributing to the intergenerational transmission of disease risk. Epigenetic modifications caused by smoking may also increase the susceptibility to respiratory diseases, cardiovascular disease, and other smoking-related health issues.
24. Explain the concept of “epitranscriptomics” and its relevance in epigenetics.
Answer: Epitranscriptomics refers to the study of chemical modifications that occur on RNA molecules, which influence gene expression and cellular function. These modifications, such as m6A methylation, pseudouridylation, and N6-methyladenosine, affect RNA stability, splicing, translation, and degradation. Epitranscriptomics is considered an extension of epigenetics because these RNA modifications are reversible and play a critical role in regulating gene expression at the post-transcriptional level. Dysregulation of RNA modifications has been linked to various diseases, including cancer, neurological disorders, and immune diseases. Understanding how RNA modifications influence gene expression is crucial for developing novel therapeutic strategies that target the epitranscriptome.
25. What is the connection between epigenetics and the microbiome?
Answer: The microbiome, which refers to the community of microorganisms living in and on our bodies, has a profound impact on the host’s epigenome. Microbial metabolites and signals can influence the expression of genes in the host through epigenetic mechanisms. For example, short-chain fatty acids (SCFAs) produced by gut bacteria can regulate gene expression by modifying histone acetylation patterns, influencing immune function, and promoting anti-inflammatory responses. Dysbiosis, or an imbalance in the microbiome, has been linked to epigenetic changes that contribute to diseases like obesity, inflammatory bowel disease, and even cancer. This connection between the microbiome and epigenetics highlights the importance of maintaining a healthy gut microbiota for overall well-being.
26. How does the study of epigenetics offer new insights into human evolution?
Answer: The study of epigenetics offers new insights into human evolution by showing how changes in gene regulation, rather than mutations in the DNA sequence, can contribute to the evolution of traits and behaviors. Epigenetic modifications can influence how genes are expressed in response to environmental factors, potentially leading to phenotypic changes that can be inherited. These modifications can provide a flexible, adaptive mechanism for evolution, allowing organisms to respond rapidly to environmental changes without requiring genetic mutations. Understanding epigenetic variation and inheritance patterns can shed light on human evolutionary history and the adaptive responses that have shaped human development and health.
27. What role do epigenetic modifications play in drug resistance?
Answer: Epigenetic modifications can contribute to drug resistance by altering the expression of genes involved in drug metabolism, transport, and the cellular response to therapy. For example, cancer cells may undergo DNA methylation or histone modifications that silence tumor suppressor genes or activate drug resistance genes. These changes can make the cells less responsive to chemotherapy or targeted therapies. In addition, non-coding RNAs and other epigenetic regulators can modulate the expression of genes involved in drug resistance. Understanding the role of epigenetics in drug resistance can help develop strategies to overcome resistance and improve the effectiveness of treatments, especially in cancer and infectious diseases.
28. What are the potential applications of epigenetic biomarkers in personalized medicine?
Answer: Epigenetic biomarkers are powerful tools in personalized medicine, as they can provide insights into an individual’s susceptibility to diseases, predict treatment responses, and guide therapeutic decisions. For example, DNA methylation patterns can serve as biomarkers for early cancer detection, as specific genes may be hypermethylated in tumors. Additionally, epigenetic profiles can help identify individuals who may respond better to certain drugs based on the epigenetic regulation of genes involved in drug metabolism. By tailoring treatments to an individual’s epigenetic profile, personalized medicine can improve the effectiveness of therapies while minimizing adverse effects, leading to more precise and individualized healthcare.
29. What are the challenges in studying epigenetics and its clinical applications?
Answer: Studying epigenetics and its clinical applications presents several challenges. One major challenge is the complexity and dynamic nature of the epigenome, as epigenetic marks can vary between cell types, tissues, and developmental stages. Additionally, the reversible and context-dependent nature of epigenetic modifications makes it difficult to pinpoint causal relationships between epigenetic changes and disease. Another challenge is the lack of standardized methods for measuring and interpreting epigenetic modifications, which can complicate the development of reliable biomarkers. Finally, the ethical implications of epigenetic research, particularly in areas such as gene editing and epigenetic inheritance, pose additional hurdles that must be carefully addressed.
30. How can environmental exposures during pregnancy influence epigenetic changes in the offspring?
Answer: Environmental exposures during pregnancy, such as diet, stress, toxins, and endocrine-disrupting chemicals, can significantly influence the epigenetic programming of the developing fetus. These exposures can lead to changes in DNA methylation, histone modifications, and non-coding RNA expression, which can affect the development of the fetus and potentially lead to long-term health consequences. For example, maternal smoking has been shown to alter DNA methylation patterns in the fetus, increasing the risk of developmental disorders and diseases later in life. These epigenetic changes may be passed on to future generations, affecting the health and disease susceptibility of offspring and even grandchildren. Understanding how environmental exposures affect the fetal epigenome is crucial for developing strategies to improve maternal and child health.